Potential drugs against flesh-eating bacteria | Washington University School of Medicine in St. Louis
Visit the News Site
It can fight serious infections that are resistant to antibiotics, a study in mice shows
 Zongsen Zou
Zongsen Zou
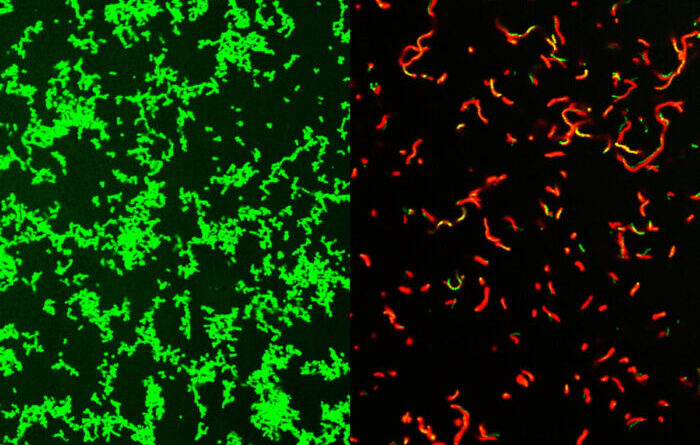
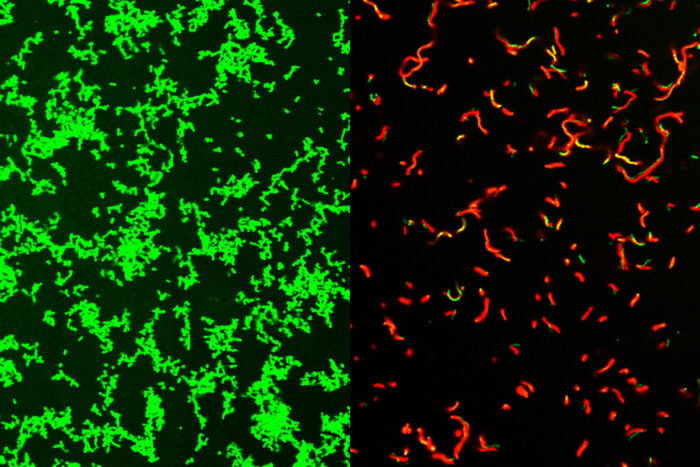
Researchers at Washington University School of Medicine in St. Louis have developed potential drugs that are effective against common bacteria that can lead to rare, dangerous diseases. Shown on the left are untreated Streptococcus pyogenes bacteria. After treatment with the mixture, the dish is full of dead bacteria (picture on the right).
Researchers at Washington University School of Medicine in St. Louis have developed a new compound that successfully clears bacterial infections in mice, including those that can result in rare but potentially fatal “flesh-eating” diseases. The potential drug could be the first of a new class of antibiotics, and a boon to doctors looking for treatments that work against bacteria that cannot be easily controlled by current antibiotics.
Research published Aug. 2 in Science Advances.
This combination targets gram-positive bacteria, which can cause drug-resistant staph infections, toxic shock syndrome and other potentially fatal infections. It was developed in collaboration between the Washington University laboratory of Scott Hultgren, PhD, Helen L. Stoever Professor of Molecular Microbiology, and Michael Caparon, PhD, professor of molecular microbiology, and Fredrik Almqvist, professor of chemistry. At the university. of Umeå in Sweden.
A new type of antibiotic could be very good news for nurses looking for an effective treatment against dangerous bacteria that are resistant to current drugs.
All the gram-positive bacteria that we tested were susceptible to that compound. That includes enterococci, staphylococci, streptococci, It’s difficultwhich are the main types of pathogenic bacteria, “said Caparon, co-author. “The compounds have a broad activity against many bacteria.”
It is based on a type of molecule called ring-fused 2-pyridone. Initially, Caparon and Hultgren had asked Almqvist to create a compound that could prevent bacterial films from sticking to the surface of urethral catheters, which is a common cause of hospital-related infections. Finding that the resulting compound has anti-infective properties against many types of bacteria was a happy accident.
The team named their new family of compounds GmPcides (for gram-positive-icide). In previous work, the authors have shown that GmPcides can eliminate bacterial strains in petri dish experiments. In this latest study, they decided to examine it with necrotizing soft tissue infection, a rapidly spreading infection that often involves a wide variety of gram-positive bacteria, which Caparon had he already has a working mouse model. The best known of these, necrotizing fasciitis or “flesh-eating disease,” can destroy tissue so quickly that it may require amputation of limbs to control its spread. About 20% of patients with carnivorous disease die.
This study focuses on one pathogen, Streptococcus pyogenes, responsible for 500,000 deaths each year worldwide, including meat-eating disease. Infected mice are S. pyogenes and GmPcide-treated animals outperformed untreated animals in nearly every metric. They had less weight loss, fewer infected wounds, and they fought off infections more quickly.
This combination appeared to reduce the virulence of bacteria and, remarkably, accelerate the postinfection healing of damaged areas of the skin.
It is not clear how GmPcides accomplishes all this, but a little analysis shows that the treatment seems to have a major effect on the bacterial membrane, which is the outer wrapping of the bacteria.
“One of the functions of the membrane is to exclude foreign substances,” said Caparon. “We know that within five to ten minutes of treatment with GmPcide, the membrane begins to penetrate and allows substances that should normally be excluded to enter the bacteria, which provides with the feeling that the membrane is damaged.”
This can interfere with bacterial activity, including those that cause damage to the host, and make the bacteria less effective in fighting the immune system against infection.
In addition to their antibacterial activity, GmPcides seem unlikely to lead to drug-resistant strains. Experiments designed to create resistant bacteria have found very few cells that can withstand the treatment, thereby passing their benefits on to the next generation of bacteria.
Caparon explained that there is a long way to go before GmPcides can find its way into local pharmacies. Caparon, Hultgren and Almqvist own the compound used in the study and have licensed it to a company, QureTech Bio, in which they have a stake, with the expectation that they will be able to collaborate with a company that has the ability to control. drug development and clinical trials that could bring GmPcides to market.
Hultgren said that the type of collaborative technology behind GmPcides is exactly what is needed to treat intractable problems like antimicrobial resistance.
He said: “Infection with viruses of all kinds is an important health problem, and resistance to many drugs is increasing, so it is difficult to treat. “The science of li -interdisciplinary facilitates the integration of different fields of study that can lead to new ideas that have the potential to help patients.”
#Potential #drugs #flesheating #bacteria #Washington #University #School #Medicine #Louis